Origin of renewable energy sources [ctd.]
1.5 Osmosis power
We have already discussed four major forms of ocean energy. All of them were well-known sources. Except ocean thermal energy, other three types represent kinetic energy of water in the ocean. In this article, we will focus on another form of ocean energy: It is osmosis power!
It does not represent kinetic energy of water in the ocean and therefore, nobody can identify its existence with the naked eyes.
It is sort of a latent energy and majority of the people have never heard of it!
What is osmosis power?
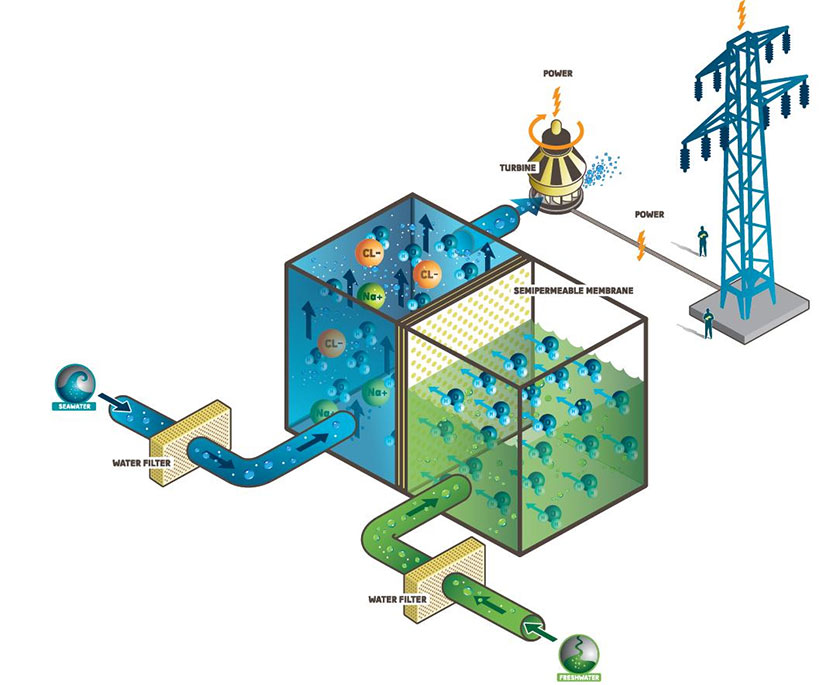
Osmosis power relies on the difference in salt concentrations (salinity gradient) between two water bodies which meet at the same place. Usually, brackish or brine water and fresh river water are the two resources of choice.
The places where rivers meet the ocean are ideal for osmosis power plants.
As we know it, brine water is extremely saltier than fresh river water and therefore, there is a sizeable difference in the salinity of water where a river meets the ocean. When fresh river water mixes up with brine water it loses its osmotic pressure where a significant amount of energy is lost. This untapped energy could be harnessed to generate electricity. Osmosis power plants are sometimes known as salinity gradient power plants since this technology relies on the difference in the salinity (salinity gradient) of two water bodies.
Osmosis power attracted much attention during the energy crisis in the 1970s. But the perseverance largely waned with the falling oil price that happened in the early 1980s.
Osmosis power (blue energy) is still under its development stage and is an infant among all other forms of oceanic energy.
A typical osmosis power plant makes use of the osmosis pressure difference between two water bodies having different salt concentrations. They transform the osmosis pressure into a hydraulic pressure which is then used to turn a turbine attached to a generator.
Several technologies such as pressure retarded osmosis, reversed electrolysis and capacitive method have been already developed to generate electricity from osmosis pressure. However, none of the technology is uneconomically viable, unfortunately. It is still at its development stage.
Technology
Usually, an osmosis power plant employs semi permeable membrane by which water having two different salt concentrations are separated. The semi permeable membrane allows water molecules to pass through it. But it does not allow ions to go through it. It is, of course, an unfair treatment!!!
But plays an essential role in osmosis power harvesting.
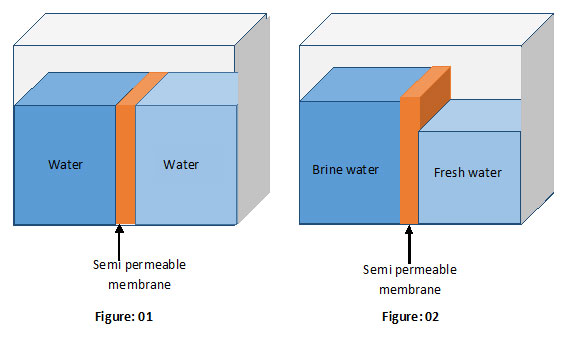
Say, you have fixed a semi permeable membrane in a storage tank as shown in the above figures. The membrane separates the tank into two parts.
We know that chemistry of materials always tries to equalize the ion concentrations of two materials in contact via a natural process called diffusion. Ions flow from the high-concentrated area to the low-concentrated area and eventually, ion concentrations in both sides become equal. But as mentioned earlier, semipermeable membranes used in osmosis power plants do not allow ions to pass through them but allow water molecules.
The consequence is amazing!
Say, you fill both chambers of the tank with the same liquid as shown in figure 1. You would see no change in the liquid levels with time.
But… What will happen if we fill two chambers with two different liquids (Liquids with two different concentrations)?
You may probably expect no change in the liquid levels with time though concentrations are different. If so, you are wrong.
The actual result definitely would surprise you!
Take a look at figure 2. It illustrates what happens if two partitions are filled with liquids having two different concentrations.
Isn’t it astonishing to see?
How does it happen?
This unusual effect is a result of the diffusion. As mentioned earlier, diffusion of matter always tries to balance the ion concentrations of two liquids in contact. This is done by transferring the ions from high concentrated are to the low-concentrated area. But now we have installed a semi permeable membrane. It prevents the transition of ions from one chamber to the other. However, the same semi permeable membrane permits the water molecules to pass through it. Consequently, the membrane promotes the transition of water molecules from low-concentrated to high concentrated. This process reduces ion concentration in the high concentrated area while increasing concentration in the low-concentrated area. Eventually, the ion concentrations in both areas become equal.
You cannot observe the changes in ion concentrations in either side with your naked eye. But there is a curious, physically observable effect. It is nothing but what you can see in figure 2.
An increase in the water level at the high-concentrated area and a drop in the water level at low-concentrated area!
Obviously, it must occur since the semi membrane prevents the transition of ions but not water molecules. This process ultimately leads to a gap between the water levels (See figure 2).
A gap between the water (liquid) levels means a hydraulic pressure!
Osmosis power plants use this hydraulic pressure to turn a turbine and generate electricity.
Some important facts
However, none of the present-day technologies offers economically practicable opportunity to harness energy from this renewable source that has concealed in water bodies.
Hopefully, it would become an economically feasible in near future at least in highly saltier areas such as the Dead Sea, red sea and Atlantic Ocean [1, 2].
The worldwide osmosis power potential is approximately 2.6 TW [3]. It is higher than the worldwide wave power potential (2 TW) [4]. But it represents only 15% of the current global energy demand even at its full capacity and would further reduce to 4.1% by 2100 (Note that the global power demand would be 63 TW by 2100) [5].
[5] Hu, A., Levis, S., Meehl, G. A., Han, W., Washington, W. M., Oleson, K. W., and Strand, W. G. (2016). Impact of solar panels on global climate. Nature Climate Change, 6 (3), 290-294.
